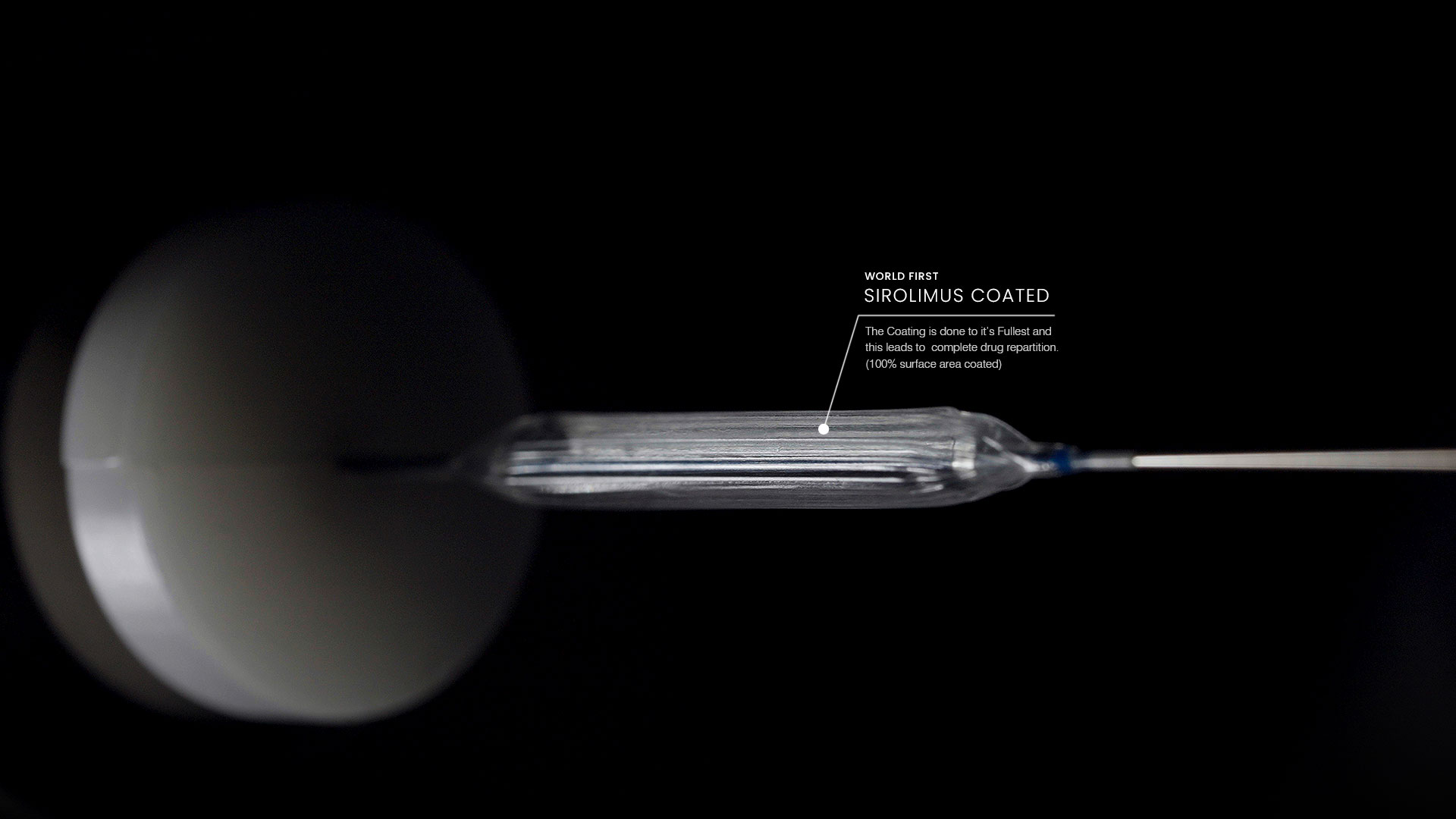
Driving Global Healthcare Innovation through Advanced Manufacturing
Our manufacturing facility is a state-of-the-art complex, designed to support the production of groundbreaking drug delivery devices. With proprietary technology and a global footprint, Envision Scientific has built an unmatched reputation for quality and precision. We cater to more than 60 countries, delivering life-saving medical products that meet the highest standards in the industry. Our success lies in our unique blend of innovation, quality control, and scalability, allowing us to produce over one million drug delivery devices annually
Global Standards, Uncompromised Quality
Meeting the Highest Compliance and Certification Benchmarks for Medical Devices
-
ESG Certified
Envision Scientific is ESG Certified! Our unwavering commitment to sustainability and responsible practices has earned us this prestigious recognition.

-
IGBC Certification
Our manufacturing units have proudly achieved the prestigious IGBC Gold rating, showcasing our unwavering commitment to sustainability and eco-friendly practices.

-
ISO Certified
Our ISO-certified manufacturing facility is equipped with state-of-the-art machinery and staffed by a dedicated workforce. Our units comply with ISO 9001:2015, ISO 13485:2016, EN ISO 13485:2016, and ISO/IEC 17025:2017 standards.

-
The EMS Monitors Rooms 24/7
The Environment Monitoring System tracks temperature, Relative humidity, and differential pressure 24/7.

-
Class 100 Cleanroom
Our manufacturing unit features a Class 100 cleanroom, ensuring the highest level of air purity for sensitive production processes.
-
Full IPQC Checks
Our manufacturing unit conducts comprehensive IPQC (In-Process Quality Control) checks to ensure the highest quality standards.

-
16CF & 24CF sterilizers, 21 CFR compliant
Our manufacturing unit is equipped with 16CF and 24CF sterilizers and is fully compliant with 21 CFR regulations, ensuring top-notch sterilization standards.

-
ICMS
Our Integrated Quality Management System (QMS) is robustly designed to meet international standards and regulatory requirements, ensuring consistent quality and compliance.

Trusted Across Continents
Delivering Advanced Drug Delivery Systems to 60+ Countries Worldwide
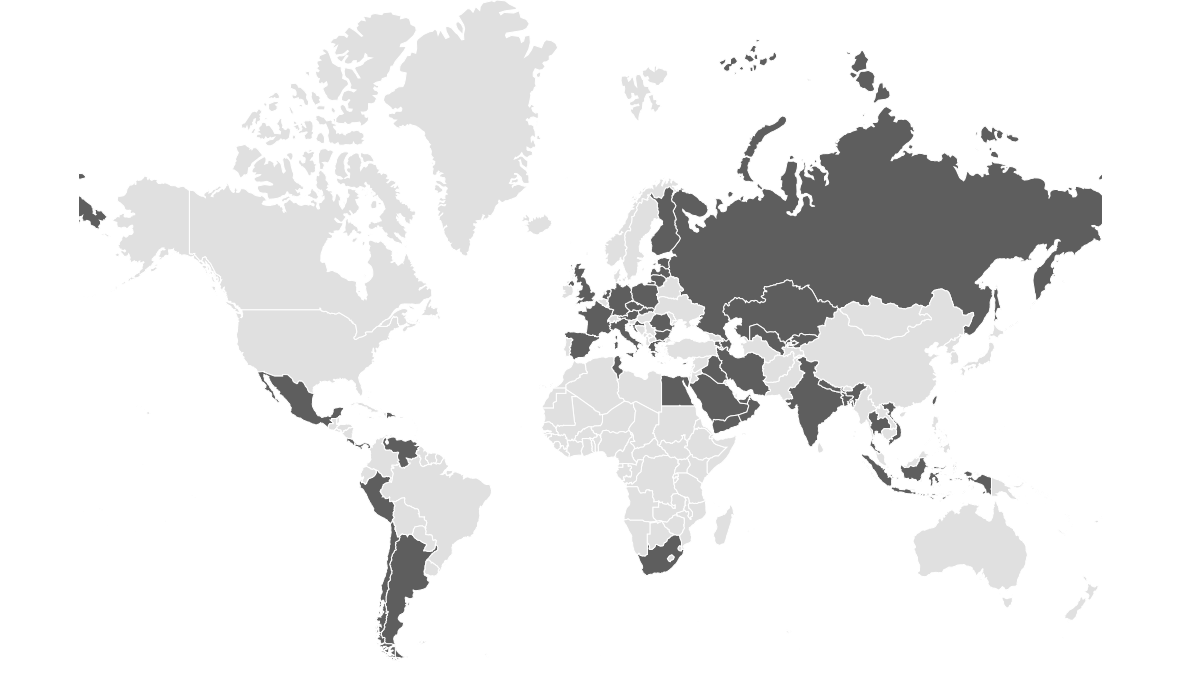
Note: Product availability countries are filled with gray [ ] color.